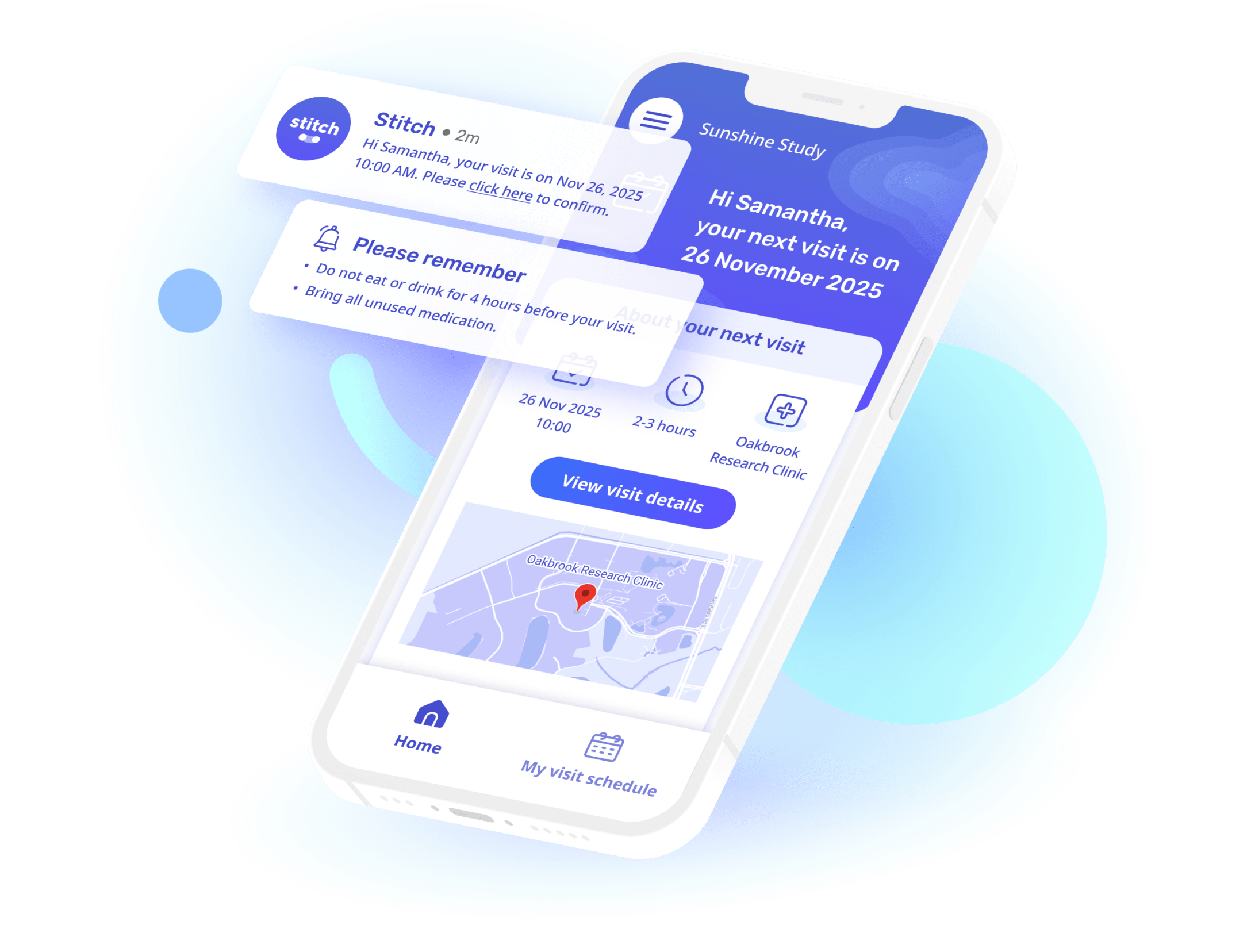
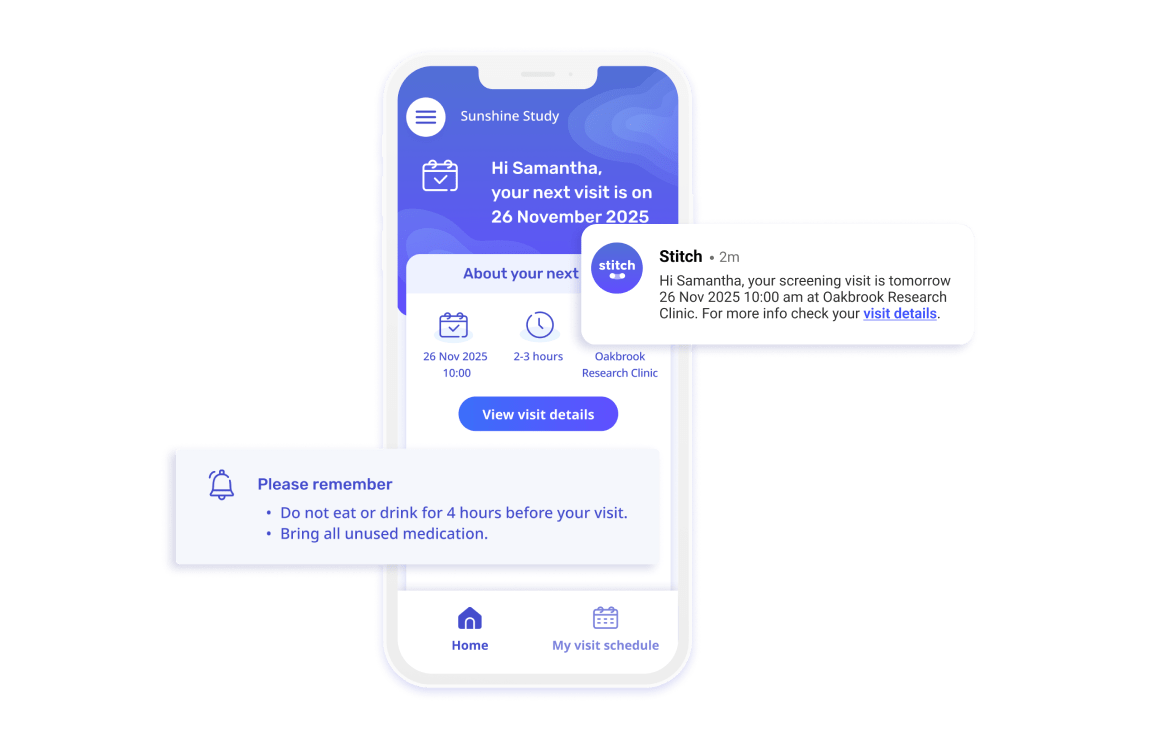
Stitch gets you more enrolled participants
18%more enrollment
27%fewer no shows
83%less admin time
36%better ePRO compliance
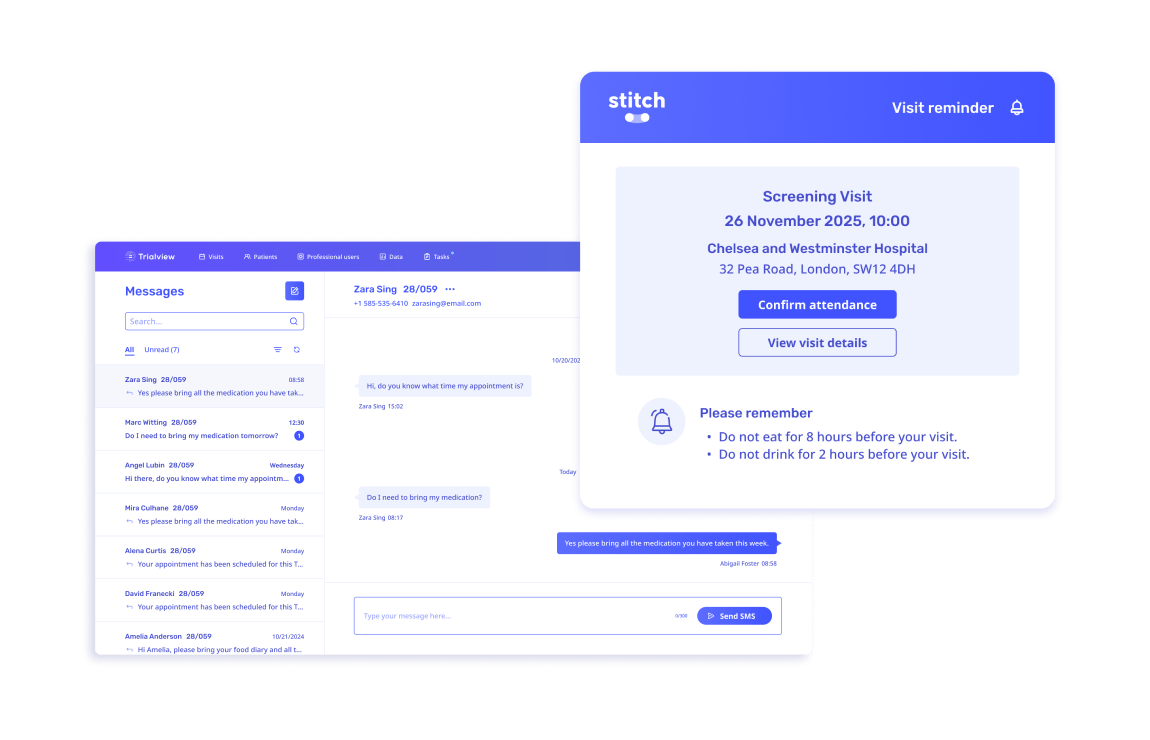
"Stitch gives our participants a user interface that pulls clinical research into this decade; with beautiful design, useful information about their visit, and the ability to give feedback in a seamless platform.
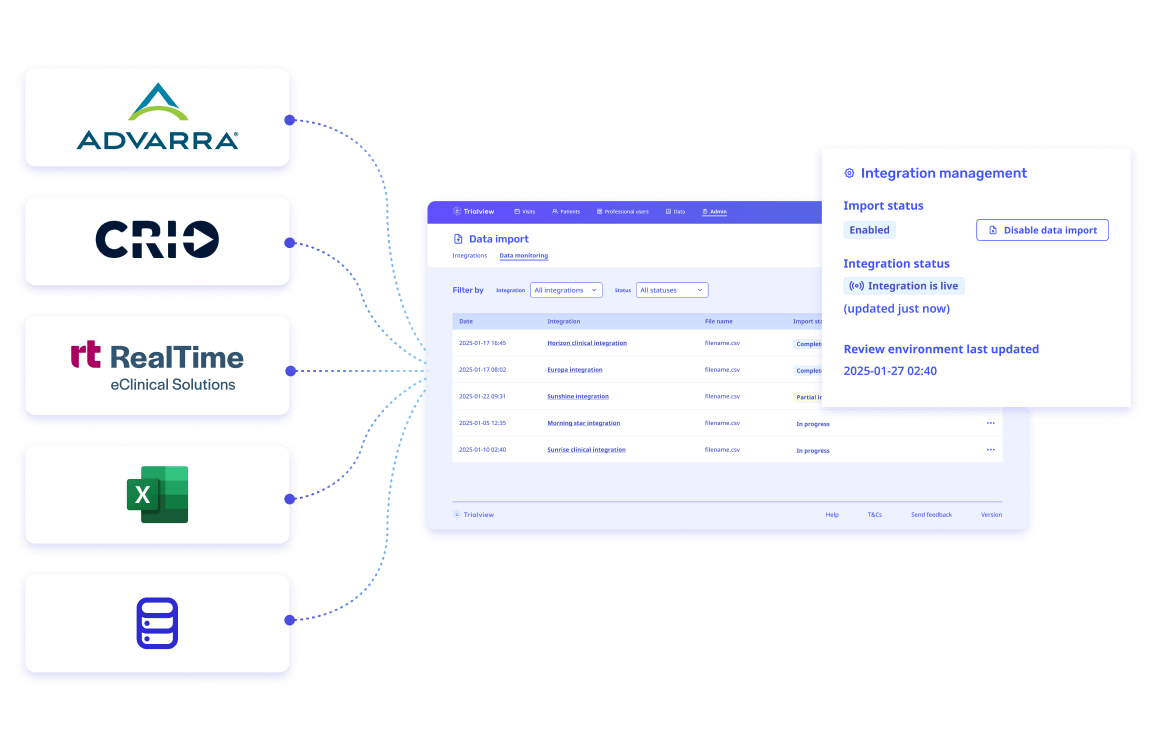
The best part is that workload has been REDUCED on our coordinators and administrative team by adding this system to our tech stack. Manual appointment reminders are now a thing of the past."


- Luke Snedaker
CEO, West Clinical Research



 © 2026 Stitch Health Ltd.
© 2026 Stitch Health Ltd.