I’ve spoken to hundreds of Clin Ops professionals, and two challenges come up time and time again - how do I find participants, and how do I keep them on my trial? Find & keep.
The “find” side of the equation is well understood. Go to any conference, and you’ll see a sea of recruitment company booths offering a wide range of approaches to finding participants to put into your screening process. But what happens from there?
If you identify 100 participants, this will on average lead to 7 participants completing your trial (source). That’s 93% of the participants you’ve found, and not kept.
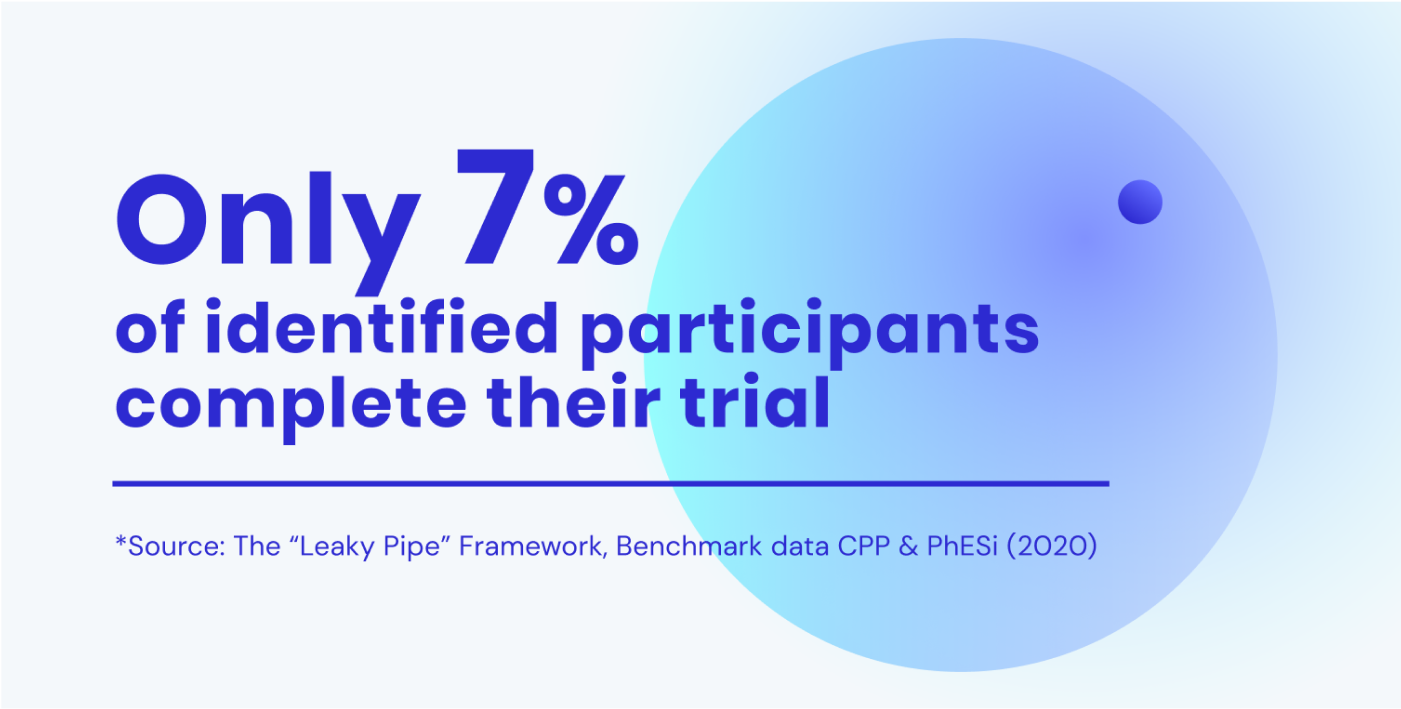
How patient experience impacts your trial performance
At Stitch, we believe that the biggest untapped opportunity in clinical trials today is a deep understanding of each patient's experience. Potential participants factor in their experience with your trial when making decisions about whether to join or remain in the study. Imagine you were offered a clinical trial, and when you asked for more information, you were given this:

Well this was received by my Co-Founder Mike’s father, when he was on his hospital bed, and was being asked to join a Phase 3 clinical trial for a top 10 pharma company. If this was happening on your trial, would you even know? Or would you just wonder why recruitment was behind plan?
That’s why companies that invest in their patient experience will improve recruitment, retention and engagement - and run faster studies.
How to invest in the patient experience
We’ve received feedback from hundreds of patients, and they wanted digital support to navigate their trial journey, like they have in other areas of their life. This includes information, visit reminders, and support to stay compliant with the trial protocol. Providing this support directly improves the patient experience, and should be table stakes in this digital age. But we can go further than this.
Retaining engaged users is not a challenge that is unique to our industry. Our founding team has overseen nearly $1bn spent on finding and keeping customers in the consumer space. What these companies do well is obsess over their customer experience. They do this in many ways, but distilling it down to its core, they listen.
We can do the same in clinical trials.
There has been a rise in recent years of patient advocacy groups being engaged before the trial is run, and their input being used to shape the trial protocol. This is fantastic, and research shared at SCOPE Europe 2023 suggests that this can save $1m+ of cost by reducing the number of protocol amendments. But why stop there?
There’s a wealth of input that you could be getting from the potential and actual participants on your trial. Clinical trials are large, complicated projects - and issues like the one shown above are bound to happen. But if you are treating your trial like a customer experience, you would be requesting feedback directly from those in your recruitment process and on your study. You can use this feedback to identify issues like this, and improve them in real time. This means that as your studies progress, they get better.
So to invest in your patient experience, you should start by listening to those in your trial.
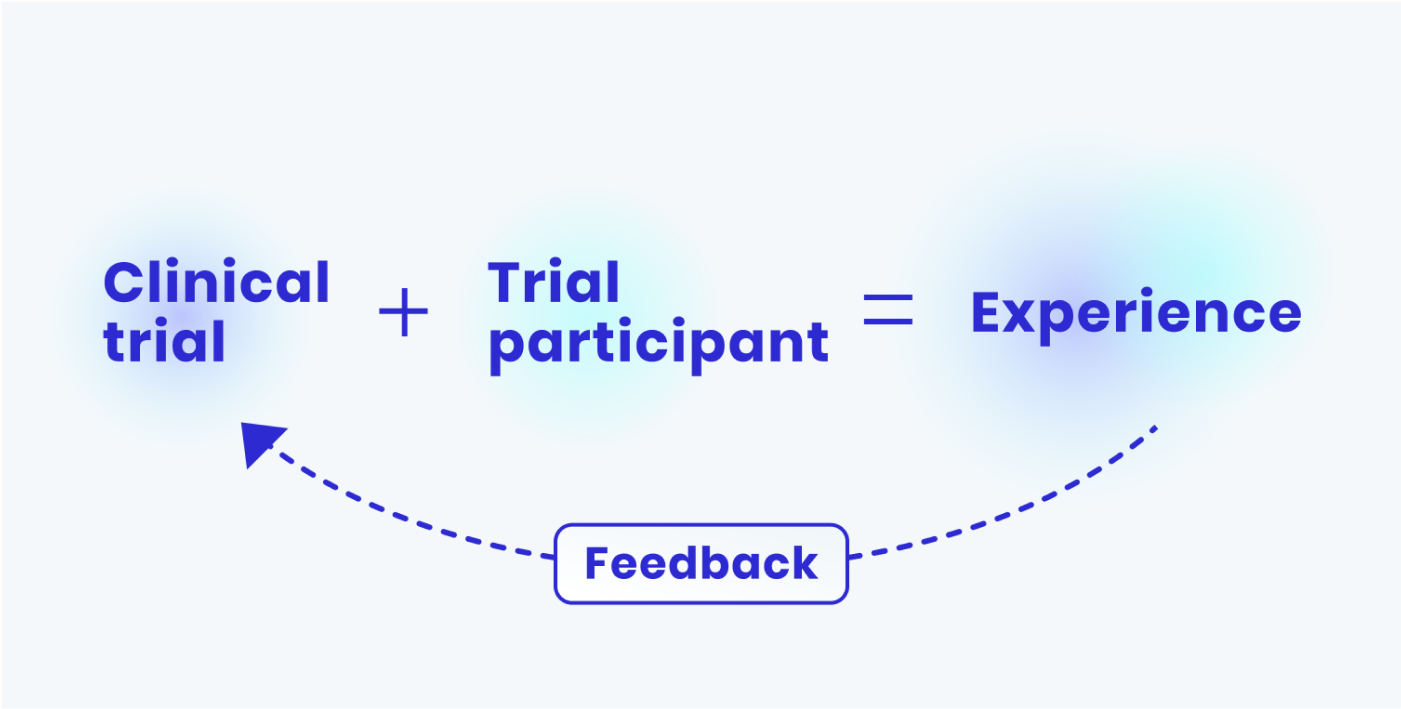
Why isn’t this done more often?
Historically, it’s been difficult to get feedback directly from participants. Only a handful of sponsors invested in the complex operational challenge of sending paper questionnaires to sites to be shared with participants, then getting them sent back, then collating and analysing the data received.
Now with the advance of technology, everyone has the opportunity to get feedback directly from participants. This can be in context of where they are in the trial, without any additional burden to sites, and without the operational complexity of doing this with paper questionnaires.
Companies that invest in this now will get huge value, and a competitive advantage in their development timelines. So how are you listening to participants on your trials?


 © 2026 Stitch Health Ltd.
© 2026 Stitch Health Ltd.